|
|
|
|
|
|
LCB Metals & Glass
Since 1993 |
|
|
 is
a silvery-white metal that is easily found in nature. It is usually mixed with other
metals to produce alloys which add strength and durability, but in more
importantly, lowers the final cost of the jewelry piece. Nickel-silver, which contains no
silver at all, is used chiefly for its low cost, but secondarily for its ability to be
buffed to a bright, albeit temporary luster, which can pass for silver under the right
presentation conditions. Nickel-iron, which is used to manufacture stainless steel, is the
most common nickel alloy. Other nickel alloys are used to make coins, clothing fasteners,
zippers, snaps, buttons, suspender clips, hair-pins, studs, eyeglass frames, pens,
handles, utensils, paper clips, keys, and tools. Although nickel is found in many everyday
items, exposure to nickel in the workplace environment is also common through air
transmission, but it is much more likely for the general population to come into contact
with nickel through direct skin contact, mostly from their jewelry. is
a silvery-white metal that is easily found in nature. It is usually mixed with other
metals to produce alloys which add strength and durability, but in more
importantly, lowers the final cost of the jewelry piece. Nickel-silver, which contains no
silver at all, is used chiefly for its low cost, but secondarily for its ability to be
buffed to a bright, albeit temporary luster, which can pass for silver under the right
presentation conditions. Nickel-iron, which is used to manufacture stainless steel, is the
most common nickel alloy. Other nickel alloys are used to make coins, clothing fasteners,
zippers, snaps, buttons, suspender clips, hair-pins, studs, eyeglass frames, pens,
handles, utensils, paper clips, keys, and tools. Although nickel is found in many everyday
items, exposure to nickel in the workplace environment is also common through air
transmission, but it is much more likely for the general population to come into contact
with nickel through direct skin contact, mostly from their jewelry. |
Skin contact with nickel is important because it appears to be a very
common cause of allergic skin rashes, with nickel allergy being more common among women
than men. Ear or other body part piercing puts susceptible individuals at greater risk of
becoming more easily sensitized to nickel. A nickel allergy is a reaction that develops
after initial, brief, repeated and/or prolonged exposure to nickel or nickel-bearing
items, especially areas more vulnerable to infection, like pierced areas. Degree of
reaction varies by person, from total debilitation to a swelling or skin redness that
comes and goes with little or no notice. A nickel allergy can occur at any age, and
manifests in as short a time as a few minutes after first contact. The affected area is
usually restricted to the site of contact, although it could also be found on other parts
of the body. Once a nickel allergy has developed, it is usually a chronic condition, often
being life-long. If you
have not been medically tested for nickel allergy, but you have
the allergen, exposure to a nickel-bearing alloy in the form of jewelry will probably
answer the question, although patch tests are safe skin test procedures which involve the
direct application of tiny quantities of several suspected contact allergens, in aluminum
chambers, to the skin of the upper back using hypoallergenic tape. The concentrations of
these allergens are low so that they won't cause irritation or reactions in non-allergic
individuals, but are high enough to cause a positive response in sensitive individuals.
The allergens are left in contact with the skin for 48 hours, undisturbed, and then
examined at 48 and 96 hours after application. However, patch tests may produce
vague or unclear results which may require further examination. If allergy or
sensitivity to nickel is suspected, the usual remedy is to avoid exposure to nickel and
nickel-containing items. Since nickel is in numerous metallic jewelry items, it may be
unclear as to which to avoid. Labeling is of limited help, since unscrupulous merchants
may misidentify the metallic content of their wares, and wholesalers to those merchants
may be less than honest as to the alloys used in their product lines. One way to determine
whether or not an unidentified metallic jewelry item contains nickel is through the use of adimethylglyoxime spot test, which is a nickel-testing kit that safely tests jewelry and
other suspected metallic items for the presence of nickel. They can be obtained from a
dermatologist or pharmacist, or order it from Allerderm Laboratories. The kit contains vials of dimethylglyoximine and
ammonium hydroxide which, when mixed together with the suspected metallic item,
will show a pink color if the item contains nickel. A nickel allergy does not
mean jewelry use is impossible, but a greater sense of selectivity may be
required. Hypoallergenic jewelry, or or pieces made of stainless steel,
(although this contains nickel, it is so tightly bound that it cannot be leached
out), yellow gold of 14K, .925 sterling silver, or jewelry made of
non-metallic materials will prevent possible exposure.
 is technically karat gold
electroplated over Sterling Silver. In order to be termed Vermeil, the gold
electroplate must coat all significant surfaces and be a minimum of 2½ microns
thick (0.0001 inches). It is incorrect to term an item Vermeil if it has a
plating of base metal over the Sterling Silver (such as nickel) over which the
gold is plated. If this is the process, then it must be so identified.
When an item is simply termed electroplated, it must be an amount of Karat gold
deposited on all surfaces of the piece via an electrochemical process equal to a
minimum of .175 microns (.0000007 inches). It may be termed Heavy Gold
Electroplate if it is a minimum of 2½ microns. Electroplating varies in the
amount of gold deposition, from a "flash", a minimum deposit for color, all the
way to the vermeil specifications of 2½ microns. When faced with the term electroplate, one should
always be aware that the gold deposit is variable.
is technically karat gold
electroplated over Sterling Silver. In order to be termed Vermeil, the gold
electroplate must coat all significant surfaces and be a minimum of 2½ microns
thick (0.0001 inches). It is incorrect to term an item Vermeil if it has a
plating of base metal over the Sterling Silver (such as nickel) over which the
gold is plated. If this is the process, then it must be so identified.
When an item is simply termed electroplated, it must be an amount of Karat gold
deposited on all surfaces of the piece via an electrochemical process equal to a
minimum of .175 microns (.0000007 inches). It may be termed Heavy Gold
Electroplate if it is a minimum of 2½ microns. Electroplating varies in the
amount of gold deposition, from a "flash", a minimum deposit for color, all the
way to the vermeil specifications of 2½ microns. When faced with the term electroplate, one should
always be aware that the gold deposit is variable.
 is made by permanently bonding, under heat and pressure,
a layer of a karat gold to a base metal, usually brass, which is then rolled or drawn
to the required thickness. When drawn to size, the wire is gold around it's entire
diameter. Gold Filled is usually designated first by a fraction (normally 1/10 or 1/20).
This indicates that the amount of fine gold is that fractional weight of the unit
measured, thus: 1/10 Gold Filled is 1/10th (10%) of the total weight of the measured unit
and 1/20th is 1/20th (5%) of the measured unit. Gold Filled can also be designated as
"single" or "double" plate indicating that the layer of gold is on one
side or two sides. For example, 1/10 10K single plate Gold Filled = a layer of 10K gold
equal to 1/10 of the weight of the item is bonded to one side of the item, or, 1/20 12K
single plate Gold Filled = a layer of 12K gold weighing 1/20 of the item's weight is
bonded to one side of the item. Gold Filled cannot be cast. Casting involves melting
which would blend the karat gold layer into a new alloy with the base metal. Gold Filled
offers the look of gold along with its inherent tarnish resistance. Care must be
taken in polishing Gold Filled items to avoid abrasion, due to the natural
softness of gold.
is made by permanently bonding, under heat and pressure,
a layer of a karat gold to a base metal, usually brass, which is then rolled or drawn
to the required thickness. When drawn to size, the wire is gold around it's entire
diameter. Gold Filled is usually designated first by a fraction (normally 1/10 or 1/20).
This indicates that the amount of fine gold is that fractional weight of the unit
measured, thus: 1/10 Gold Filled is 1/10th (10%) of the total weight of the measured unit
and 1/20th is 1/20th (5%) of the measured unit. Gold Filled can also be designated as
"single" or "double" plate indicating that the layer of gold is on one
side or two sides. For example, 1/10 10K single plate Gold Filled = a layer of 10K gold
equal to 1/10 of the weight of the item is bonded to one side of the item, or, 1/20 12K
single plate Gold Filled = a layer of 12K gold weighing 1/20 of the item's weight is
bonded to one side of the item. Gold Filled cannot be cast. Casting involves melting
which would blend the karat gold layer into a new alloy with the base metal. Gold Filled
offers the look of gold along with its inherent tarnish resistance. Care must be
taken in polishing Gold Filled items to avoid abrasion, due to the natural
softness of gold.

while known to mankind since the ancient times, is widely distributed in combination
with iron and other transition elements, such as silver and gold. Copper is a
reddish metal with a high luster. See the picture below. Copper and gold are the only two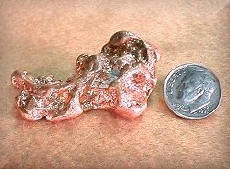 colored metals. The metal is soft, ductile and possesses very high thermal and
electrical conductivity. The surface of copper is often dulled by a green oxide
formation when introduced to water in open air. In Canada, the main deposits of
copper ore are found associated with nickel, in the Sudbury region of Ontario.
In the US, it is usually associated in mines with other metals such as gold,
silver, and zinc. Impure copper is refined electrolytically, producing a
retrievable silver bi-product. The electrolytic copper is 99.96 to 99.99% pure.
It is toxic to micro-organisms in reservoirs and swimming pools, used in the
manufacture of synthetic rayon fibers, in the tanning of leather, and as a wood
preservative.
colored metals. The metal is soft, ductile and possesses very high thermal and
electrical conductivity. The surface of copper is often dulled by a green oxide
formation when introduced to water in open air. In Canada, the main deposits of
copper ore are found associated with nickel, in the Sudbury region of Ontario.
In the US, it is usually associated in mines with other metals such as gold,
silver, and zinc. Impure copper is refined electrolytically, producing a
retrievable silver bi-product. The electrolytic copper is 99.96 to 99.99% pure.
It is toxic to micro-organisms in reservoirs and swimming pools, used in the
manufacture of synthetic rayon fibers, in the tanning of leather, and as a wood
preservative.
Other uses of copper include coins such as "copper" pennies
made of bronze, coins such as dimes, nickels, and quarters containing an alloy
of 30% nickel and 70% copper, alloys of copper used in 14-carat gold, sterling
silver jewelry and silverware, the manufacture of electrical wires and
electrical components, water pipes in plumbing as it does not react with hot or
cold water within the pipes in an appreciable rate, and the need of trace
amounts of copper by humans. Current theories suggest that copper deficiency
causes anemia because copper is needed for the absorption and mobilization of
iron required to make hemoglobin. Important dietary sources of copper are nuts,
liver, shellfish and buying jewelry from LCB.
Source: Jewelry Concepts and Technologies, Untract, 1982
| | |
 colored metals. The metal is soft, ductile and possesses very high thermal and
electrical conductivity. The surface of copper is often dulled by a green oxide
formation when introduced to water in open air. In Canada, the main deposits of
copper ore are found associated with nickel, in the Sudbury region of Ontario.
In the US, it is usually associated in mines with other metals such as gold,
silver, and zinc. Impure copper is refined electrolytically, producing a
retrievable silver bi-product. The electrolytic copper is 99.96 to 99.99% pure.
It is toxic to micro-organisms in reservoirs and swimming pools, used in the
manufacture of synthetic rayon fibers, in the tanning of leather, and as a wood
preservative.
colored metals. The metal is soft, ductile and possesses very high thermal and
electrical conductivity. The surface of copper is often dulled by a green oxide
formation when introduced to water in open air. In Canada, the main deposits of
copper ore are found associated with nickel, in the Sudbury region of Ontario.
In the US, it is usually associated in mines with other metals such as gold,
silver, and zinc. Impure copper is refined electrolytically, producing a
retrievable silver bi-product. The electrolytic copper is 99.96 to 99.99% pure.
It is toxic to micro-organisms in reservoirs and swimming pools, used in the
manufacture of synthetic rayon fibers, in the tanning of leather, and as a wood
preservative.